In the last decades, the European regulatory landscape has experienced a significant transformation. The main goal of this evolution has been to harmonize European standards, facilitate intra-EU trade, and promote innovation within the sector. However, this process has also faced challenges, particularly due to the coexistence of European frameworks and national regulations.
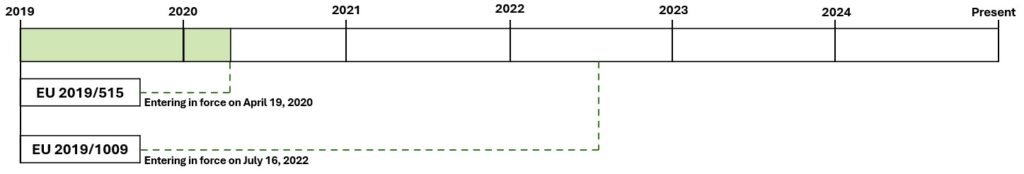
First Period: Before April 19th 2020
Regulation 2003/2003: Context and Functioning
Regulation (EC) No. 2003/2003, adopted on October 13th 2003, established a harmonized framework for inorganic fertilizers in the European Union, allowing their free movement under the “EC Fertilizer” label.
Key Features of Regulation 2003/2003:
Scope: Only regulated and focused on inorganic fertilizers.
Objectives:
- Ensure the quality and safety of fertilizers in the EU market.
- Establish minimum nutrient content requirements.
- Define clear and precise labelling standards.
Technical Requirements:
- Fertilizers had to meet minimum nutrient values (nitrogen, phosphorus, potassium).
- Detailed specifications for composition, labelling, and quality testing.
Limitations of the Regulatory Framework: Despite its role in harmonising the inorganic fertiliser market, Regulation 2003/2003 had significant limitations such as not including organic fertilisers, biostimulants and other innovative products. It was also the case that products not regulated at European level were subject to national legislation. This situation created a fragmented market, making cross-border trade for non-harmonised products very difficult.
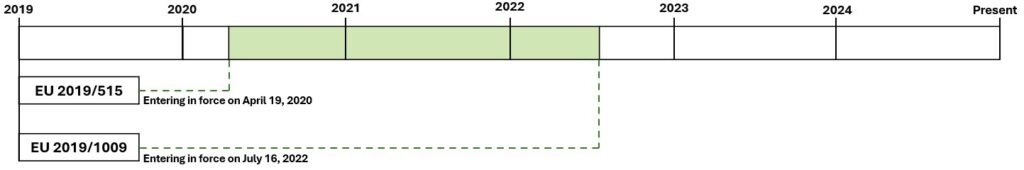
Second Period: From April 19th 2020 to July 16th 2022
Introduction of Regulation 2019/515 on Mutual Recognition
Regulation (EU) 2019/515, approved on March 19th 2019, entered in force on April 19th 2020, with the main goal of reinforcing the principle of mutual recognition within the EU single market.
Key Principles of Regulation 2019/515:
- If a product (fertilizers, biostimulants, etc.) is legally marketed in one Member State, it must be allowed to be marketed in other Member States.
- It applied to non-harmonized products at the EU level (those not covered by regulation 2003/2003).
- Introduced a clear mechanism for evaluating and resolving disputes in cases where a Member State refused to recognize a product.
Coexistence of Regulations: During this period, three regulatory frameworks coexisted.
- Regulation 2003/2003: Continued to apply to inorganic fertilizers.
- National Regulations: Each country maintained its own legislation for products not covered by Regulation 2003/2003.
- Regulation 2019/515: Facilitated cross-border trade through mutual recognition.
Application and Benefits of Mutual Recognition: Regulation 2019/515 allowed companies to market innovative products (such as biostimulants) in multiple countries without duplicating registration processes. It also reduced trade barriers caused by differing national regulations. However, some Member States implemented justified restrictions (for example, environmental or safety reasons), which partially limited the effectiveness of mutual recognition.
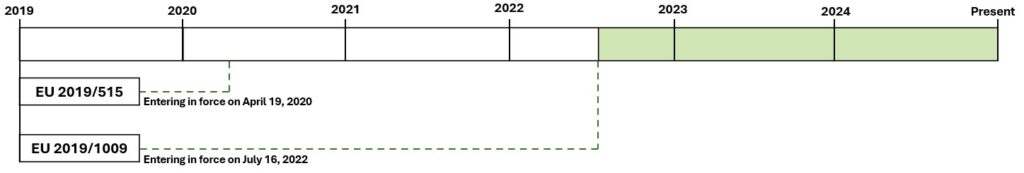
Third Period: From July 16th 2022 to Present
Entry into Force of Regulation 2019/1009
Regulation (EU) 2019/1009, approved on June 5th 2019, came into force on July 16th 2022, gradually replacing Regulation 2003/2003.
Key Innovations of Regulation 2019/1009:
- Expanded the scope to include Inorganic and Organic Fertilizers, Biostimulants, Growing Media and Soil Improvers.
- Introduced the CE Mark for these products, facilitating their free movement within the EU.
- Established specific technical criteria for biostimulants, defining their function and safety requirements.
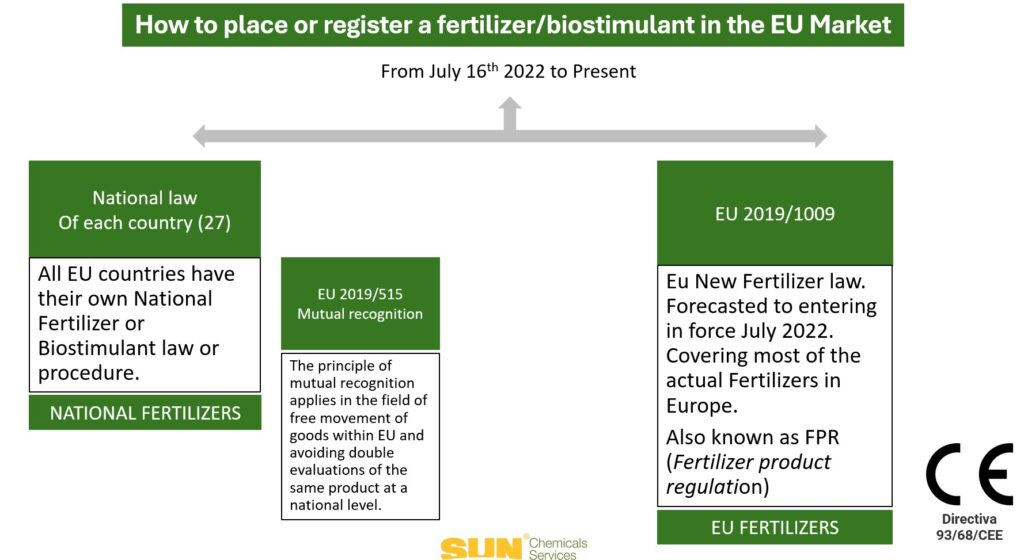
Complementarity with Regulation 2019/515 and National Laws: Coexisting at the moment are Regulation 2019/1009 (main harmonised framework for fertiliser products), Regulation 2019/515 (continues to apply for non-harmonised products under Regulation 2019/1009) and national laws (allow for exclusive marketing in local markets).
Advantages and Challenges of the Current Framework
Advantages:
- Greater harmonization for innovative products (Such as biostimulants).
- Reduction in trade barriers.
Challenges:
- Persistence of national requirements for non-harmonized products.
- Complexity for companies needing to choose between EU or national regulations.
The evolution of the regulatory framework in Europe has allowed for greater harmonization and flexibility, especially with the introduction of Regulation 2019/1009. However, the coexistence of different regulations and the application of mutual recognition continue to pose challenges. The future of this regulatory landscape will depend on further simplification and adaptation to the needs of the market and innovation.
January 2025
Sun Chemicals Services Team
#Fertilizers #Bioestimulants #EuropeanRegulations #2003/2003 #2019/515 #2019/1009 #EuropeanRegistration


