European agriculture entered a new phase since the entry into force of Regulation (EU) 2019/1009 in July 2022, which replaced the former EC Regulation 2003/2003. This regulatory change brought about a significant transformation in the regulatory framework for fertiliser products, especially with respect to biostimulants.
What has changed?
Under the old regulation, only inorganic fertilisers could be marketed. However, the new regulation opened the door to a wide range of products such as organic fertilisers, biostimulants, growing media and soil improvers.
One of the most relevant developments is that, for the first time, it is legally defined what a biostimulant is and what its functions are.
The definition is as follows: “plant biostimulant: a product stimulating plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere:
a) nutrient us efficacy
b) tolerance to abiotic stress
c) quality traits
d) Availability of confined nutrients in soil or rhizosphere”
Efficacy trials
In order to register a biostimulant under this legislation, it is mandatory to demonstrate its efficacy through field trials. These trials must be carried out on representative groups of crops, categorised by the European Commission in three main groups.
- Broadacre crops: These include annual or multi-annual crops grown over large areas, harvested with machinery. Examples: lentils, oats, potatoes, soybeans, sesame, rice, cotton, etc.
- Woody perennials: Crops that are not renewed annually and whose stems are hardened with bark. They include almond trees, apple trees, olive trees, vines, avocado trees, mango trees, etc.
- Horticultural, ornamental and aromatic and medicinal plants (AMP): These include both annual and perennial crops not included in the other categories: tomato, lettuce, carrots, spinach, rosemary, etc.
The trials must demonstrate that the biostimulant improves one or more of the claimed functions. This is an essential step to ensure that the product complies with its labelling.
How many trials are required?
The minimum number of trials required depends on the intended scope of the product.
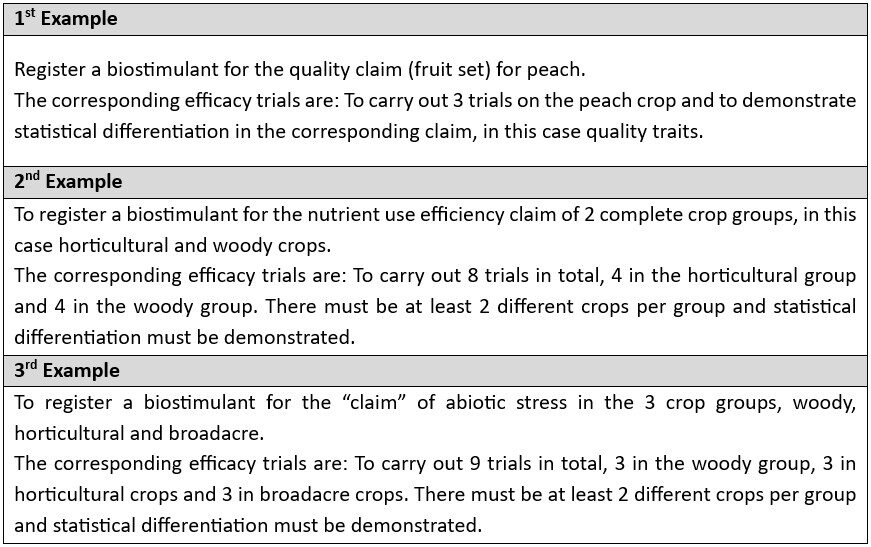
- If the effect on a specific crop is claimed, 3 tests must be performed on that crop.
- If the effect is to be claimed for a whole crop group (e.g. all woody crops), 6 trials must be carried out. These 6 trials must be carried out on at least 2 different crops of the group.
- If the effect is to be claimed for 2 complete groups, 8 trials must be performed, 4 per group, with at least 2 different crops per group.
- If the effect is claimed for all groups, 9 trials must be performed, distributed in a way that there will be 3 trials per group with at least 2 different crops per group.
This structure ensures a representative and solid evaluation.
At Sun Chemicals Services, we support companies that want to launch biostimulants on the European market. From the design of efficacy trials, through the interpretation of results, to the preparation of regulatory dossiers. We offer comprehensive advice to facilitate compliance with Regulation 2019/1009.
May 2025
Sun Chemicals Services Team
#Biostimulants #Fertilisers #EfficacyTrials #Regulation2019/1009 #Crops


